White Paper
Mechanism of Colitis induction by Dextran Sodium Sulfate (DSS)
Jan 2025 – Nasim Najjarzadeh (PhD), Marketing Manager
Inflammatory Bowel Disease (IBD)
IBD, including Crohn’s disease and ulcerative colitis, results from a dysregulated immune response in the gastrointestinal tract. Despite extensive research, the exact cause remains elusive, involving genetic and environmental factors. Understanding the mechanisms behind this inflammatory response is crucial for developing effective treatments.
Animal Models in IBD Research
Animal models, particularly those using dextran sodium sulfate (DSS), have been indispensable in IBD research. These models provide valuable insights into the histopathological and morphological changes in the intestinal tract, helping researchers unravel the disease’s pathogenesis and identify potential therapeutic targets.
Colitis induction by DSS in animal models
Dextran Sodium Sulfate (DSS) Colitis Model
DSS, a water-soluble, negatively charged sulfated polysaccharide with a molecular weight of 35-50 kDa, is used to induce colitis in mice. The DSS colitis model is favored for its rapidity, simplicity, reproducibility, and controllability. By adjusting DSS concentration and administration frequency, researchers can create models of acute, chronic, and relapsing inflammation (1).
Recent studies using murine colitis models have enhanced our understanding of IBD pathogenesis, focusing on:
- Intestinal Barrier Disruption: Investigating how the integrity of the intestinal barrier is compromised and its role in disease progression.
- Mucin’s Role: Examining the impact of mucin on colitis development and severity.
- Microbial Balance Alterations: Exploring changes in gut microbiota and their contribution to inflammation and disease.
- Metabolome Changes: Analyzing shifts in the gut metabolome and their implications for IBD pathogenesis.
Key Factors in the Pathogenesis of IBD
Mechanism of DSS-Induced Colitis
DSS acts as a chemical toxin to the colonic epithelium, causing epithelial cell injury and disrupting the intestinal epithelial monolayer. This disruption allows luminal bacteria and antigens to enter the mucosa, triggering an inflammatory response. The effectiveness of DSS-induced colitis depends on factors such as DSS concentration (1%-5%), duration and frequency of administration, molecular weight, animal strain, and microbial environment.
Typically, 35-50 kDa DSS is added to sterilized drinking water to achieve the desired inflammatory effect. At this molecular weight, DSS effectively penetrates the mucosal membrane. Larger (520 kDa) and smaller (10 kDa) molecular weights fail to induce colitis due to inadequate tissue penetration.
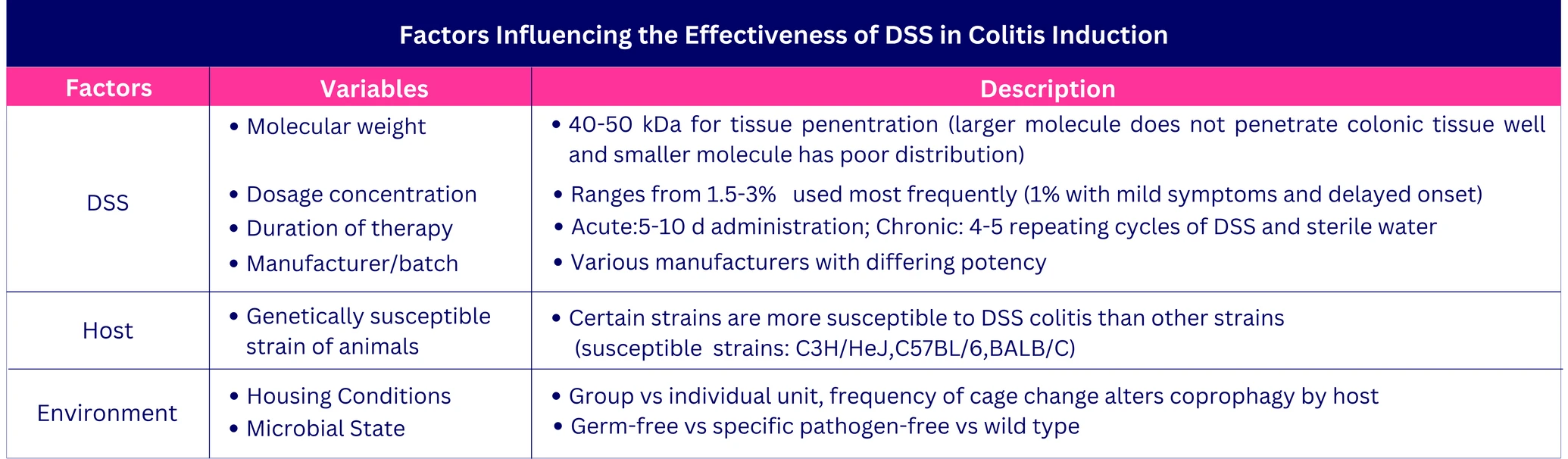
Factors influencing the effectiveness of DSS on Colitis induction
Acute vs chronic colitis
Acute colitis in mice is induced by administering DSS for 6-10 days, resulting in weight loss, diarrhea, and hematochezia. Chronic colitis is induced through 4-5 repeated cycles of DSS, each cycle consisting of one week of DSS administration followed by 7-14 days of sterile water. The severity of colitis can be modulated by adjusting the duration and concentration of DSS.
DSS administration triggers disease signs within a day, marked by changes in tight junction proteins and increased epithelial apoptosis, leading to barrier leaks seen in human IBD. Symptoms worsen over time, including increased intestinal permeability, severe bleeding, and mortality.
Acute DSS causes mucin and goblet cell depletion, epithelial erosion, ulceration, and granulocyte infiltration, triggering immune responses. Chronic DSS leads to crypt architecture disarray, widening of the crypt-muscularis gap, deep mucosal lymphocytosis, and neutrophil migration, resulting in cryptitis and crypt abscesses. Prolonged DSS can cause squamous metaplasia, adenomatous changes, and adenocarcinoma.
General protocol to induce acute colitis by DSS:
General protocol to induce acute colitis by DSS
General protocol to induce chronic colitis by DSS:
General protocol to induce chronic colitis by DSS
Carcinogenesis
The DSS model is used to study colitis-associated cancer (CAC). Combining DSS with azoxymethane (AOM) induces chronic colitis and dysplasia, mimicking human ulcerative colitis. The transition from inflammation to cancer involves immune response, oncogene activation, tumor suppressor inhibition, and changes in intestinal microbiota.
The AOM/DSS model is the most widely used murine model for studying CAC. Long-term or repeated DSS administration induces chronic colitis and dysplasia in rodents. In this model, inflammation correlates with dysplasia and is associated with β-catenin activation, enhancing the Wnt signaling pathway and increasing pro-inflammatory cytokines (IL-6, TNF-α). However, typical human CAC mutations like Kras or p53 are not present in this model (1).
Differences in Inflammatory Profiles
DSS colitis increases cytokine and chemokine production, starting on the first day. Key mediators include TNF-α, IL-6, IL-10, IL-17, IL-1β, and TGF-β. Acute inflammation transitions to a Th-2 response in chronic states. These profiles correlate with barrier function and clinical parameters, making DSS valuable for studying cytokine roles in inflammation.
Mechanism of DSS action:
- DSS disrupts intestinal barrier function: DSS disrupts the intestinal barrier by altering tight junction proteins, leading to increased permeability and inflammation.
- DSS effects on mucin: DSS affects mucin production and function, compromising the mucus barrier and contributing to colitis.
- DSS alters the microbial balance: DSS changes the gut microbiota composition, influencing inflammation and disease progression.
- dextran sodium sulfate-induced changes in metabolome: DSS alters the gut metabolome, affecting metabolic processes and contributing to IBD pathogenesis.
1. DSS Disrupts Intestinal Barrier Function
The intestinal epithelium acts as a selective barrier, allowing nutrient, ion, and water absorption, regulated by tight junction (TJ) complexes consisting of occludin, ZO, claudins, and JAM (junctional adhesion molecules). Dysfunction in this barrier increases intestinal permeability, linked to inflammatory bowel disease (IBD). In IBD, changes in TJ protein expression and function, along with poorly adherent mucosa, permit bacterial entry and reduce transepithelial resistance. Studies show that TJ alterations and increased permeability often precede inflammation. In mice, DSS colitis causes a decrease in TJ expression, leading to increased permeability and colonic inflammation. Therefore, TJ complex changes are a prerequisite for the development of intestinal inflammation.
Studies showed that the protein pattern of TJs undergoes rapid changes. Claudin proteins, a family of 27 genes, play a crucial role in forming the intestinal epithelial cell (IEC) barrier. Most claudins provide barrier properties, especially in the tight epithelia of the distal intestine. They interact in a tissue-specific way to create charge- and size-selective barriers, significantly contributing to the epithelial barrier function and regulating paracellular permeability. Studies have shown varying results regarding the changes in the expression of different claudins in relation to DSS administration. For instance, the expression of claudin-1 protein varied across different studies, which could be attributed to the use of different experimental animal species, such as rats or mice. Findings have shown an up-regulation of the pore-forming claudin-2, along with decreased expression of claudin-3, -5, -7, and -8 (2).
Studies proved the critical role of claudin-2 in maintaining colonic epithelial homeostasis and barrier function, thereby influencing mucosal immune response and inflammation. During inflammation, claudin-2 expression often increases and integrates into TJ strands, starting in the lower crypt and moving toward the surface epithelium. The functional significance of this altered expression and the resulting increase in intestinal permeability remains unclear (3). Similarly, human studies on IBD patients have shown a significant increase in claudin-2 expression (4). In DSS-treated Cl-2TG mice, claudin-2 overexpression significantly suppressed pro-inflammatory molecules, highlighting its role in immune adaptation. Increased regulatory CD4+ cells in unchallenged mice and reduced immune cell infiltration in DSS-challenged mice suggest that claudin-2-induced epithelial permeability promotes adaptive tolerance and protection from colitis by facilitating the interaction of host immune molecules and luminal antigens (4). Additionally, DSS-challenged mice showed decreased apoptosis and increased epithelial proliferation, supporting claudin-2’s role in intestinal epithelial regulation. Conversely, claudin-2 knockout mice subjected to DSS exhibited severe colitis (4).
There is also evidence about the influence of other proteins such as ZO-1. Studies showed a significant decrease in ZO-1 after one day of DSS treatment, causing the loss of TJ integrity and therefore an increase in permeability (5). Moreover, the distribution of occludin and ZO-1 also changes after DSS administration (6).
Another adhesion molecule with essential roles in the development and homeostasis of several tissues is the large group of proteins E-cadherin (7). It is shown that mice with E-cadherin deficiency exhibited more severe colitis in DSS models (7). Additionally, similar to occluding and ZO-1, E-cadherin, and β-catenin also translocate from the junctions in the colonic epithelium after 4 days of DSS treatment.
2. DSS effects on mucin
Human studies have shown that patients with colonic inflammation experience alterations in colonic mucus and decreased barrier function effectiveness (8). The gastrointestinal tract’s protective mucus barrier consists of secretory, gel-forming mucins forming the outer loose layer and inner dense membrane-bound mucins covering the epithelial cells (8,9). Mucins are large, filamentous molecules with highly O-glycosylated peptide domains (10). Humans have fifteen different mucins in the MUC gene family, with only a few active in the colon (8,10). Muc2 is the primary secretory gel-forming mucin in both large and small intestines, providing a barrier between the epithelium and microbiota (8,9). Other membrane-bound mucins are involved in cell signaling, adhesion, growth, and immune system modulation (8,10). Defects in mucin genes or protein folding can lead to poor membrane integrity and interaction of mucose and bacteria.
In-vitro studies on intestinal cell lines show that mucin expression and structure are influenced by cytokines, bacteria, and their components. In ulcerative colitis (UC), changes in immunological or bacterial factors can affect mucin production. Goblet cell depletion, a common finding in UC patients, suggests decreased Muc2 synthesis, leading to chronic inflammation characteristic of IBD. It remains uncertain whether mucin decrease and goblet cell depletion are primary contributors to IBD or consequences of inflammation (8,10,11). Mice deficient in Muc2 lack a mucus layer, allowing bacteria to contact epithelial cells and penetrate deeper into the crypts, unlike healthy animals. These mice are more susceptible to severe colitis and have an increased risk of colon cancer (12,13,14). During DSS induction, mucus gel thickness decreases as colitis symptoms worsen (8).
Post-translational modifications are crucial for mucin functionality. Increased Muc2 levels after DSS administration fail to control inflammation due to decreased sulphation (10,11). Altered glycosylation of mucins reduces Muc2 synthesis and weakens the mucus barrier, increasing susceptibility to DSS-induced colitis (11). These findings highlight the importance of post-translational modifications in regulating intestinal barrier integrity (11). Cell surface mucins, along with secretory mucins, are crucial for mucosal protection (9,11). Muc4, a type-Ⅰ transmembrane glycoprotein, is typically found on colonic epithelial cells. Muc4-/- mice are more resistant to colitis and CAC induced by AOM/DSS due to compensatory upregulation of Muc2 and Muc3 (9). These mice also show increased expression of pro-inflammatory cytokines (TNF-α and IL-1β), which may drive this upregulation (9). Conversely, Muc13-/- mice exhibit increased inflammation and severe colitis symptoms, suggesting that Muc13 protects colonic epithelial cells from apoptosis (11). Muc4-/- mice show a protective phenotype with less severe colitis, linked to the upregulation of Muc2 and Muc3 (9,15).
3. DSS alters the microbial balance
The intestinal microbiome is crucial for immunity development, tolerance, and physiological processes. This has led to large efforts to identify and characterize microorganisms linked to health and disease (16,17,18,22). Besides the presence of microbiota intestinal tract, the composition, temporal changes, and relative abundance of specific microbes are important for homeostasis and disease states like IBD (10,18,19).
3.1. Effects on Mucosal Inflammation
The intestinal microbiome is crucial for immune development and physiological processes. DSS-induced colitis studies show that gut microflora plays a key role in mucosal inflammation, and barrier function however, the exact mechanism of bacterial-induced inflammation remains unclear (20,19 ).
Microbiome alterations can lead to normally underrepresented bacteria becoming dominant, disrupting microbiome structure and function. DSS-induced colitis studies in mice show early microbiota changes, characterized by reduced diversity and occurring before clinical inflammation signs (21,22,23). These changes include increased Bacteroidaceae and Clostridiaceae families (24) and reduced Bacteroidetes, Prevotella, Clostridium, and Lactobacillus, with increased pro-inflammatory Bacillaceae, Enterococcales, and Enterobacteriaceae (21,25,26).
3.2. Effects on Barrier Functions
Studies have shown that bacteria can influence barrier function alterations. In DSS-induced colitis, epithelial integrity is compromised, allowing microbes and antigens to penetrate the mucosa (27,22,28). Faecalibacterium prausnitzii has anti-inflammatory effects by decreasing paracellular permeability, reducing colitis severity, and preventing progression. It decreases NF-κB activation and IL-8 secretion in vitro and reduces pro-inflammatory cytokines while increasing anti-inflammatory cytokines in vivo (29). Various strains of bifidobacteria, particularly Bifidobacterium bifidum, also show anti-inflammatory effects by inhibiting LPS-dependent NF-κB activation in intestinal epithelial cells (IECs) and inducing anti-inflammatory immune cells (30).
3.3. Effects on Mucin
Metagenomics studies after DSS administration showed an increase in Akkermansia muciniphila, which metabolizes sulfur, degrades mucin, and correlates with disease activity in DSS-treated mice (21,22,25). DSS-induced colitis eliminated differences in bacterial abundance and structure between the two mucus layers (12,27). There were fewer bacteria in the firmly adherent mucus, but the mucus layer of DSS-treated animals had 10-100 times more bacteria than the control group, indicating an increase in infectious bacteria (20). Antibiotic treatment improved DSS-induced colitis, similar to reduced inflammation seen in germ-free murine colitis models (21,17).
Note:
- After stopping DSS, the gut microbiota rapidly shifts to a healthy profile, demonstrating resilience and recovery of its mutualistic state (21).
- Probiotic therapies with Lactobacilli and Bifidobacteria improve outcomes in murine colitis models by enhancing barrier integrity and modulating tight junction proteins (30,31). Specific strains such as Lactobacillus reuteri, reduce colitis severity by reducing bacterial translocation and suppressing adherence to colonic mucosa (20). The protective effects of probiotics are linked to strengthening epithelial barrier integrity rather than changes in microbial composition (20,27).
4. Dextran Sodium Sulfate-induced changes in metabolome
DSS colitis disrupts phospholipid metabolism, decreasing levels of phosphocholine and glycerophosphocholine, which are essential for membrane integrity (32). High dietary fat intake is linked to an increased risk of ulcerative colitis (33). In the large intestine, bile acids are transformed by gut microbes into secondary bile acids like deoxycholic acid and UDCA. These secondary bile acids vary in hydrophobicity and affect gut barrier function by disrupting cell membranes, causing cytotoxicity, producing reactive oxygen species, activating the epithelial growth factor receptor, and redistributing tight junctions (34). Consequently, this increases gut permeability, enhances the translocation of bacteria and associated antigens across the tight-junction barrier of the gut epithelium, and leads to inflammation. Lower fecal cholic acid levels correlate with more histologic damage (34,35). Additionally, different types of dietary fats can promote colitis by altering gallbladder bile and gut microbiota. Changes in taurocholic acid levels in bile facilitate the growth of Bilophila wadsworthia, an inflammatory Gram-negative anaerobe. Overall, the hydrophobicity of fecal bile acids, mediated by a higher proportion of deoxycholic acid, positively correlates with the severity of DSS colitis (34,36).
Reference
1). Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017 Sep 7;23(33):6016-6029. doi: 10.3748/wjg.v23.i33.6016. PMID: 28970718; PMCID: PMC5597494.
2) Yuan B, Zhou S, Lu Y, Liu J, Jin X, Wan H, Wang F. Changes in the Expression and Distribution of Claudins, Increased Epithelial Apoptosis, and a Mannan-Binding Lectin-Associated Immune Response Lead to Barrier Dysfunction in Dextran Sodium Sulfate[1]Induced Rat Colitis. Gut Liver 2015; 9: 734-740 [PMID: 25717051 DOI: 10.5009/gnl14155]
3) Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015; 3: e977176 [PMID: 25838982 DOI: 10.4161/21688370.2014.977176]
4) Ahmad R, Chaturvedi R, Olivares-Villagómez D, Habib T, Asim M, Shivesh P, Polk DB, Wilson KT, Washington MK, Van Kaer L, Dhawan P, Singh AB. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol 2014; 7: 1340-1353 [PMID: 24670427 DOI: 10.1038/mi.2014.21]
5) Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 2007; 140: 12-19 [PMID: 17418867 DOI: 10.1016/j.jss.2006.07.050]
6) Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao R. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J 2015; 465: 503-515 [PMID: 25377781 DOI: 10.1042/BJ20140450]
7) Grill JI, Neumann J, Hiltwein F, Kolligs FT, Schneider MR. Intestinal E-cadherin Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis. Dig Dis Sci 2015; 60: 895-902 [PMID: 25634675 DOI: 10.1007/s10620-015-3551-x]
8) Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 2011; 300: G327-G333 [PMID: 21109593 DOI: 10.1152/ajpgi.00422.2010]
9) Das S, Rachagani S, Sheinin Y, Smith LM, Gurumurthy CB, Roy HK, Batra SK. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene 2016; 35: 2645-2654 [PMID: 26364605 DOI: 10.1038/onc.2015.327]
10) Einerhand AW, Renes IB, Makkink MK, van der Sluis M, Büller HA, Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur J Gastroenterol Hepatol 2002; 14: 757-765 [PMID: 12169985 DOI: 10.1097/00042737-200207000-00008]
11) Sheng YH, Hasnain SZ, Florin TH, McGuckin MA. Mucins in inflammatory bowel diseases and colorectal cancer. J Gastroenterol Hepatol 2012; 27: 28-38 [PMID: 21913981 DOI: 10.1111/ j.1440-1746.2011.06909.x]
12) Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014; 63: 281-291 [PMID: 23426893 DOI: 10.1136/gutjnl-2012-303207]
13) Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006; 131: 117-129 [PMID: 16831596 DOI: 10.1053/j.gastro.2006.04.020]
14) Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002; 295: 1726-1729 [PMID: 11872843 DOI: 10.1126/science.1069094]
15) Hoebler C, Gaudier E, De Coppet P, Rival M, Cherbut C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig Dis Sci 2006; 51: 381-389 [PMID: 16534686 DOI: 10.1007/s10620-006- 3142-y]
16) Mar JS, Nagalingam NA, Song Y, Onizawa M, Lee JW, Lynch SV. Amelioration of DSS-induced murine colitis by VSL#3 supplementation is primarily associated with changes in ileal microbiota composition. Gut Microbes 2014; 5: 494-503 [PMID: 25144681 DOI: 10.4161/gmic.32147]
17) Gkouskou KK, Deligianni C, Tsatsanis C, Eliopoulos AG. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol 2014; 4: 28 [PMID: 24616886 DOI: 10.3389/ fcimb.2014.00028]
18) Brinkman BM, Becker A, Ayiseh RB, Hildebrand F, Raes J, Huys G, Vandenabeele P. Gut microbiota affects sensitivity to acute DSS-induced colitis independently of host genotype. Inflamm Bowel Dis 2013; 19: 2560-2567 [PMID: 24105395 DOI: 10.1097/ MIB.0b013e3182a8759a]
19) Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002; 20: 495-549 [PMID: 11861611 DOI: 10.1146/annurev. immunol.20.100301.064816]
20) Fujimoto J, Kadara H, Garcia MM, Kabbout M, Behrens C, Liu DD, Lee JJ, Solis LM, Kim ES, Kalhor N, Moran C, Sharafkhaneh A, Lotan R, Wistuba II. G-protein coupled receptor family C, group 5, member A (GPRC5A) expression is decreased in the adjacent field and normal bronchial epithelia of patients with chronic obstructive pulmonary disease and non-small-cell lung cancer. J Thorac Oncol 2012; 7: 1747-1754 [PMID: 23154545 DOI: 10.1097/JTO.0b013e31826bb1ff]
21) De Fazio L, Cavazza E, Spisni E, Strillacci A, Centanni M, Candela M, Praticò C, Campieri M, Ricci C, Valerii MC. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol 2014; 20: 2051-2061 [PMID: 24587679 DOI: 10.3748/wjg.v20.i8.2051]
22) Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 2011; 17: 917-926 [PMID: 21391286 DOI: 10.1002/ibd.21462]
23) Deplancke B, Finster K, Graham WV, Collier CT, Thurmond JE, Gaskins HR. Gastrointestinal and microbial responses to sulfate[1]supplemented drinking water in mice. Exp Biol Med (Maywood) 2003; 228: 424-433 [PMID: 12671187 DOI: 10.1177/153537020322800413]
24) Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990; 98: 694-702 [PMID: 1688816 DOI: 10.1016/0016-5085(90) 90290-H]
25) Håkansson Å, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt ML, Karlsson C, Jeppsson B, Cilio CM, Ahrné S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med 2015; 15: 107-120 [PMID: 24414342 DOI: 10.1007/s10238-013-0270-5]
26) Rath HC, Schultz M, Freitag R, Dieleman LA, Li F, Linde HJ, Schölmerich J, Sartor RB. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun 2001; 69: 2277-2285 [PMID: 11254584 DOI: 10.1128/ IAI.69.4.2277-2285.2001]
27) Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 2008; 105: 15064-15069 [PMID: 18806221 DOI: 10.1073/ pnas.0803124105]
28) Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun 2008; 377: 12-16 [PMID: 18796297 DOI: 10.1016/ j.bbrc.2008.09.019]
29) Carlsson AH, Yakymenko O, Olivier I, Håkansson F, Postma E, Keita AV, Söderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol 2013; 48: 1136-1144 [PMID: 23971882 DOI: 10.3109/00365521.2013.828773]
30) Grimm V, Radulovic K, Riedel CU. Colonization of C57BL/6 Mice by a Potential Probiotic Bifidobacterium bifidum Strain under Germ-Free and Specific Pathogen-Free Conditions and during Experimental Colitis. PLoS One 2015; 10: e0139935 [PMID: 26439388 DOI: 10.1371/journal.pone.0139935]
31) Srutkova D, Schwarzer M, Hudcovic T, Zakostelska Z, Drab V, Spanova A, Rittich B, Kozakova H, Schabussova I. Bifidobacterium longum CCM 7952 Promotes Epithelial Barrier Function and Prevents Acute DSS-Induced Colitis in Strictly Strain-Specific Manner. PLoS One 2015; 10: e0134050 [PMID:26218526 DOI: 10.1371/journal.pone.0134050]
32) Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 2014; 18: 279-288 [PMID: 25177159 DOI: 10.4196/kjpp.2014.18.4.279]
33) Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis 2008; 14: 1348-1357 [PMID: 18484673 DOI: 10.1002/ibd.20491]
34) Stenman LK, Holma R, Forsgård R, Gylling H, Korpela R. Higher fecal bile acid hydrophobicity is associated with exacerbation of dextran sodium sulfate colitis in mice. J Nutr 2013; 143: 1691-1697 [PMID: 24047703 DOI: 10.3945/jn.113.180810]
35) Araki Y, Andoh A, Tsujikawa T, Fujiyama Y, Bamba T. Alterations in intestinal microflora, faecal bile acids and short chain fatty acids in dextran sulphate sodium-induced experimental acute colitis in rats. Eur J Gastroenterol Hepatol 2001; 13: 107-112 [PMID: 11246608 DOI: 10.1097/00042737-200102000-00004]
36) Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat[1]induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012; 487: 104-108 [PMID: 22722865. DOI: 10.1038/nature11225].




What do you think about this website?