Dextran molecules in solution may be regarded as flexible expandable coils. However, this is somewhat of an oversimplification since the smaller molecules < 5000 Da appear to behave as a more rigid rod-like structure – which would account for the tendency to aggregate on standing. The very large molecules with longer side chains display greater symmetry.
The sizes of dextran molecules in solution can be expressed either as the radius of gyration, √R2 or as the hydrodynamic radius Rh (Stoke’s radius). These two parameters differ significantly as shown in table 1 below. Information on size and conformation is adduced from viscosity, sedimentation, and diffusion studies.
The observed values are influenced not only by the molecular weight but also by the branching, which may vary as the molecular weight increases. This is due to the cleavage of the branches on hydrolysis of the dextran and that the lower molecular weight fractions will require longer hydrolysis times than the higher fractions.
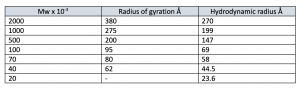
Table 1. The sizes of dextran molecules in solution. The radius of gyration or hydrodynamic radius (also called Stoke’s radius) is often used to express the size of dextran in solution.
The conformation of dextran and other polysaccharides is influenced significantly by substitution since repulsion of the charged groups will lead to an expansion of the coils. These effects will also depend on the degree of substitution and at very low levels the conformation is essentially unchanged. Thus, FITC-dextrans are identical on GPC to their parent dextran fractions. Dextran derivatives with hydrophobic substituents (e.g. phenyl- dextran) may form micelles but with GPC in pure water (to suppress hydrophobic interaction with gel), it does not appear to show anomalies. Many derivatives with charged substituents are eluted earlier, thus giving an apparently higher molecular weight.